The ISO/IEC 17025 Solution
SoftExpert offers the most advanced and comprehensive software solution for organizations and laboratories that perform testing, sampling or calibration, meeting the stringent needs of various global regulations. SoftExpert’s Excellence Suite helps organizations and laboratories comply with ISO/IEC 17025 while lowering costs, maximizing success, increasing productivity and reducing risks.
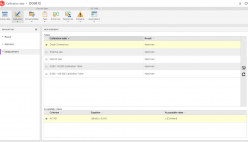
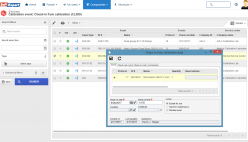
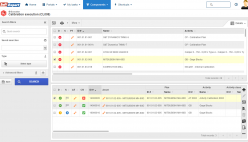
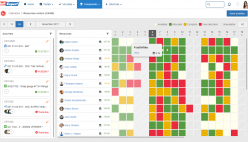
SoftExpert’s solution enables organizations to easily meet ISO/IEC 17025 requirements, providing resources to plan and implement actions to manage calibrations, risks and opportunities, confidentiality, procedures, equipment, personnel, systems, processes, and nonconformities. With online collaboration capabilities, the organization and managers can communicate and receive updates on compliance initiatives, involving more users, teams, offices and business units with a systematic and unified approach, turning quality and safety guidelines into actions.