ISO 15189 Solution
SoftExpert offers the most advanced and comprehensive software solution for compliance management, meeting the demanding needs of various global regulations. SoftExpert Excellence Suite helps companies to comply with ISO 15189 while lowering costs, maximizing success, increasing productivity and reducing risks.
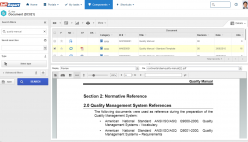
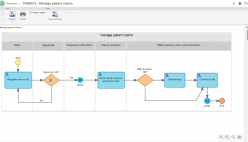
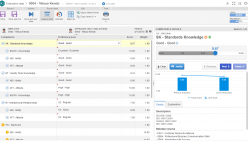
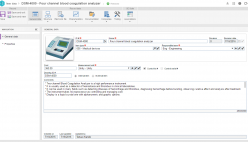
SoftExpert’s solution enables organizations to easily meet ISO 15189 requirements, providing resources to develop policies, manage complaints and nonconformities, fix targets and objectives, analyze and understand data, and measure results to make decisions regarding patient care, boosting QMS efficiency and reducing rework and waste. With online collaboration capabilities, the organization and managers can communicate and be updated on quality initiatives, involving more users, teams, offices and business units with a systematic and unified approach that ensures the maintenance and effectiveness of the QMS.