Increased productivity and efficiency
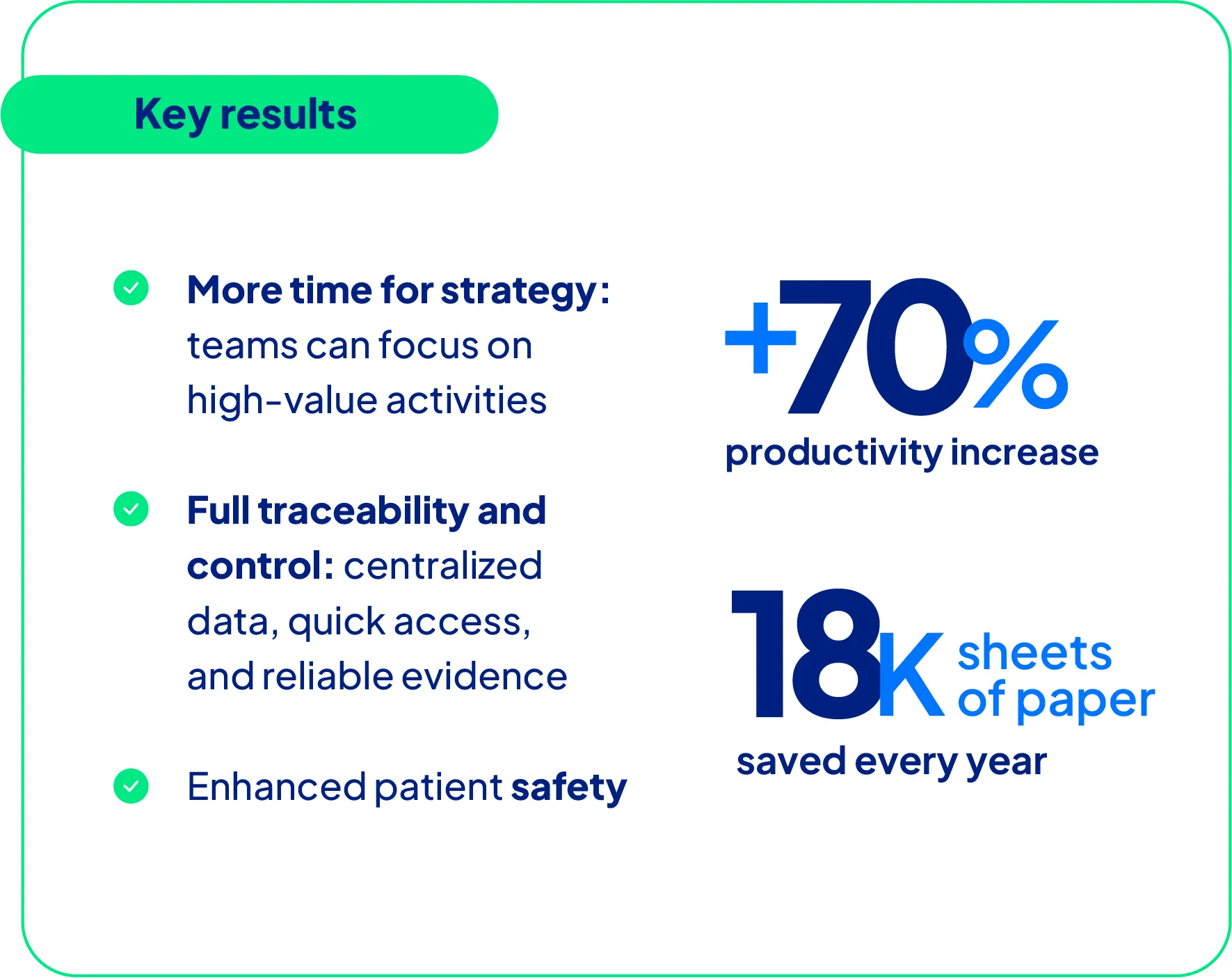
Eliminating manual steps led to a 70% increase in productivity
Optimization and compliance
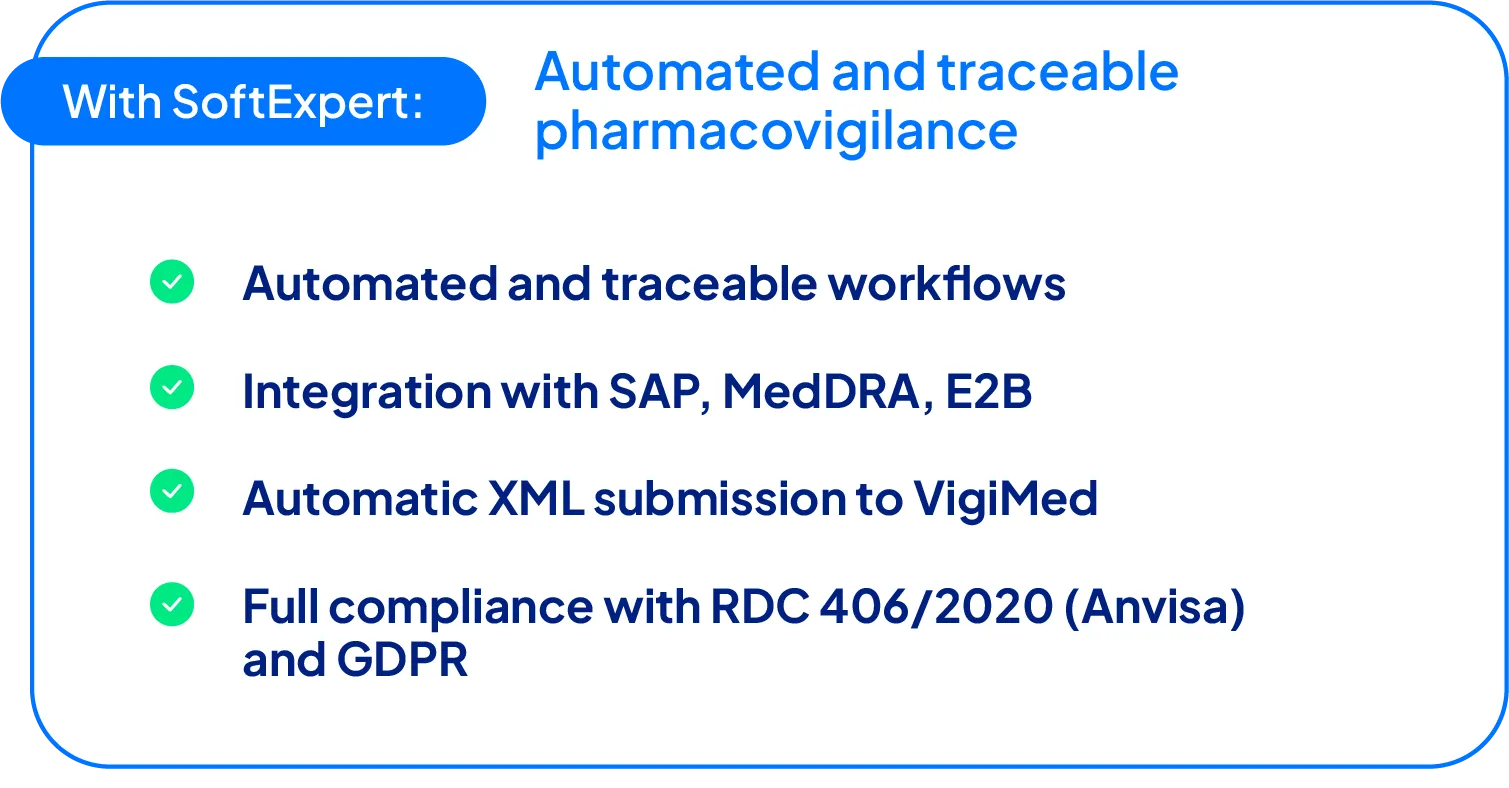
Through integration with SAP, MedDRA, and E2B, XML files are automatically transmitted to Anvisa’s Vigimed system
Proven return
Results include significant annual cost savings and the elimination of 18,000 sheets of paper each year

Geolab
Geolab is one of Brazil’s leading manufacturers of generic and similar medicines, driven by a simple and essential purpose: caring for people. Health is the company’s greatest inspiration, a value that guides every decision, innovation, and product developed to improve the quality of life of Brazilians.
With 100% national capital, Geolab Indústria Farmacêutica is based in DAIA, Anápolis (GO), the largest pharmaceutical and chemical hub in Latin America. Recognized for its consistent growth in the pharmaceutical market, the company employs more than 2,200 people and continues to invest in technology, quality, and operational excellence.
Geolab boosts productivity by 70% with SoftExpert
The Brazilian pharmaceutical company transformed its pharmacovigilance process with SoftExpert Pharmacovigilance, increasing team productivity by 70%. With seamless integration to SAP, MedDRA, and E2B, XML files are now automatically sent to Anvisa’s Vigimed system, streamlining processes while maintaining full compliance with regulatory and data protection standards.
Pharmacovigilance involves the continuous monitoring of medication safety after market release. In Brazil, it is regulated by Anvisa, while the World Health Organization (WHO) emphasizes the importance of accurate and timely reporting of adverse events. Delayed or missing reports can lead to regulatory risks, fines, or even the withdrawal of medications from the market. Efficient pharmacovigilance systems are, therefore, essential to ensure compliance and safeguard public health.
This case highlights Geolab’s journey in transforming its pharmacovigilance operations. What used to be a hybrid, error-prone process, partly manual and fragmented, became fully optimized with SoftExpert Pharmacovigilance. With an automated, traceable, and efficient system, Geolab boosted productivity, ensured data integrity, and strengthened its reputation for patient safety.
Geolab in the Pharmaceutical landscape

Geolab is one of Brazil’s leading pharmaceutical companies, specializing in generic and similar medicines. With over 2,200 employees and 100% Brazilian capital, the company has shown consistent growth, driven by continuous investment in technology, innovation, and quality.
With a portfolio of more than 400 products across 84 therapeutic classes, Geolab is expanding its operations with new facilities and strengthening its presence in ophthalmic and inhalation medicines, solidifying its position as a benchmark in the Brazilian pharmaceutical industry.
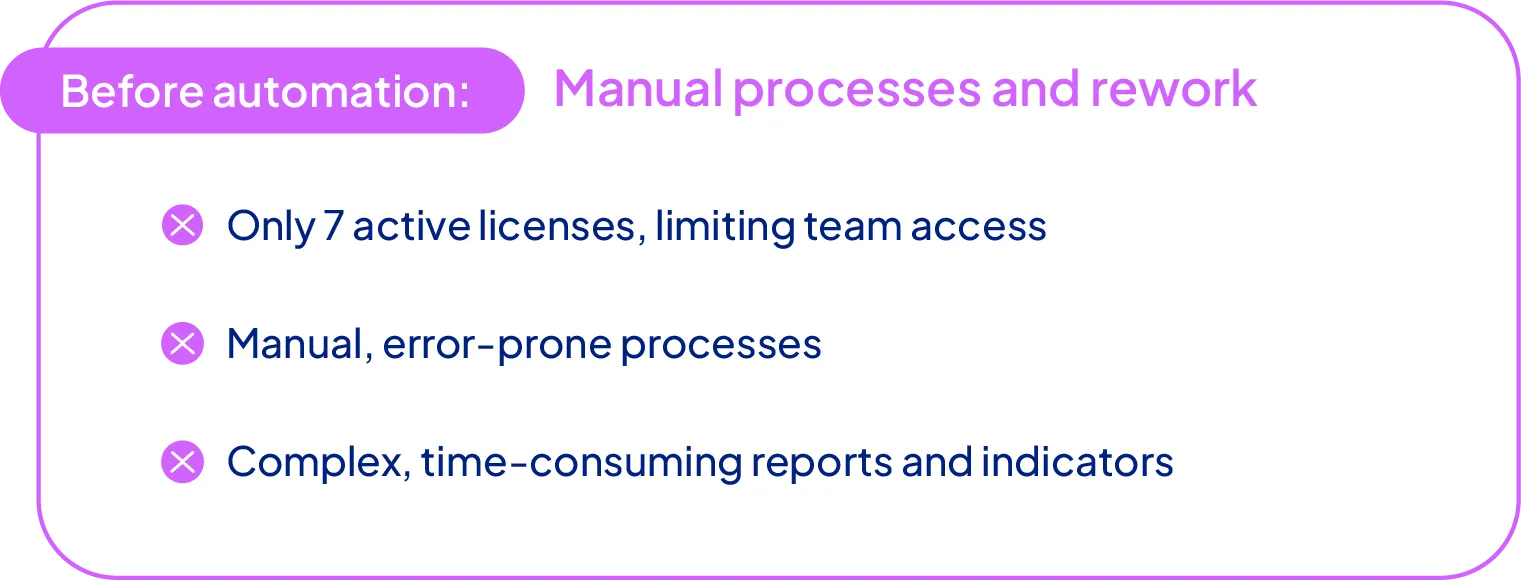
A complex starting point
Before implementing SoftExpert, Geolab’s pharmacovigilance process was entirely manual and limited. Only seven licenses were available (including the IT team), which restricted system access. Adverse event reporting required exporting data to Word, printing documents, collecting handwritten signatures, and re-entering information into the system, all of which increased the risk of errors and delays.
Generating indicators and reports was time-consuming, and the lack of automatic deadline notifications posed additional regulatory risks.
The turning point with SoftExpert
The decision to adopt a new pharmacovigilance solution was driven by management’s strategic vision: eliminate costs associated with the legacy system’s licenses and expand automation to other critical processes, such as nonconformities and CAPA (Corrective and Preventive Actions).

“We already had a solid partnership with SoftExpert, using their solutions for change control and risk analysis. That experience, combined with our trust in the platform’s stability and flexibility, was decisive in choosing them for this new project,” recalls Juliana Santana, Quality Assurance Coordinator at Geolab.
The new system was collaboratively designed by Geolab and SoftExpert, ensuring regulatory alignment and incorporating specific operational needs. One of the main challenges was integration with E2B (Electronic Transmission of Individual Case Safety Reports) from Anvisa (as required by RDC No. 406/2020), which mandates the submission of XML-based reports.
Optimization and compliance
The pharmacovigilance process, previously fragmented and manual, was unified into a single automated workflow, offering a holistic view and tighter control over each step.
One of the most notable improvements was the automatic classification of events — such as serious cases, inefficacy, or adverse reactions — directly within the system.
“Eliminating manual steps delivered a substantial gain, a 70% increase in productivity. The need to print documents, collect physical signatures, or retype data was completely eliminated. The entire workflow, from initial record to final approval, now takes place within the system, ensuring data integrity and full traceability,” adds Juliana.
A major milestone was the automatic generation of E2B XML files for Anvisa submissions. Previously, the team had to manually enter all data into Anvisa’s Vigimed system. With SoftExpert, XML files are automatically generated, downloaded, and uploaded directly to Vigimed, eliminating transcription errors and drastically reducing processing time.
Approval workflows, which previously relied on paper signatures, now occur entirely within the system, allowing reviewers or pharmacovigilance managers to return cases for correction, ensuring accuracy before submission.
Integration with SAP and MedDRA (Medical Dictionary for Regulatory Activities) also removed the need to manually type drug names or batch numbers, making processes faster and more reliable. SoftExpert fully complies with GDPR (General Data Protection Regulation), ensuring that only authorized users can access sensitive data.
Productivity and efficiency gains
The productivity gains were remarkable: Geolab reported a 70% increase in team efficiency, directly resulting from automation and the elimination of bottlenecks.
A tangible example of cost savings is the reduction of around 1,500 printed pages per month. Considering that Geolab processes about 500 reports monthly, each averaging three pages, eliminating manual printing has significantly reduced both paper use and administrative effort.
Improved traceability and information access
All data related to pharmacovigilance cases, including phone logs, WhatsApp screenshots, images, and videos, can now be uploaded directly into the system. This centralization ensures quick, reliable access to information and eliminates the need for multiple physical or digital repositories.
Geolab now benefits from robust dashboards and detailed analytics, offering productivity insights by analyst and case volume by product. These data-driven tools provide valuable intelligence for management and decision-making.
Looking ahead
The transition to SoftExpert has consolidated critical processes, strengthened product quality management, and enhanced patient safety. Driven by its results and culture of innovation, Geolab continues to evolve — with new investments underway, such as implementing the FMEA module and improving existing workflows.